The libretexts libraries are powered by mindtouch and are supported by the department of education open textbook pilot project the uc davis office of the provost the uc davis library the california state university affordable learning solutions program and merlot.
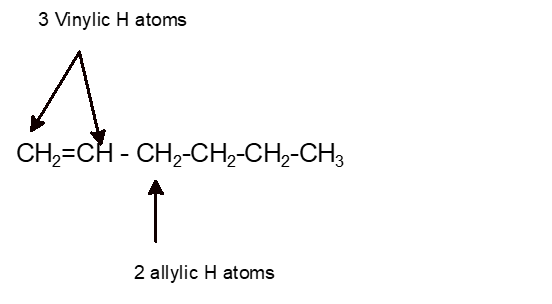
Vinylic hydrogen atoms.
An industrially important example is vinyl chloride precursor to pvc a plastic commonly known as vinyl.
Number of hydrogen atoms.
On a carbon skeleton sp2 hybridized carbons.
The name is also used for any compound containing that group namely r ch ch2 where r is any other group of atoms.
Allylic carbon can have a maximum of 3 hydrogen atoms.
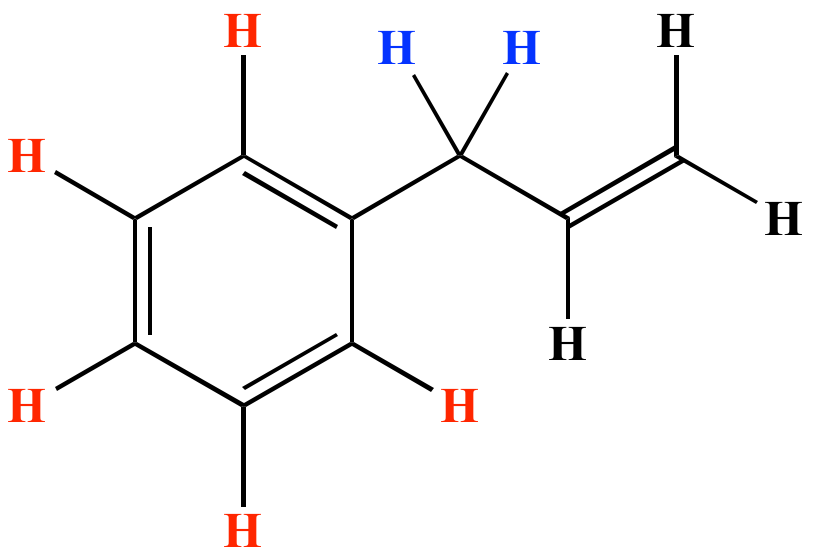
Vinyl contains two sp 2 hybridized carbon atoms and three hydrogen atoms.
We also acknowledge previous national science foundation support under grant numbers 1246120 1525057 and 1413739.
Allylic carbon only forms a single bond.
An allylic hydrogen is a hydrogen atom that is bonded to an allylic carbon in an organic molecule.
Since both carbon atoms form a double covalent bond so both are sp 2 hybridized.
The allylic position is also like a vinylic position.
It forms at least one double bond.
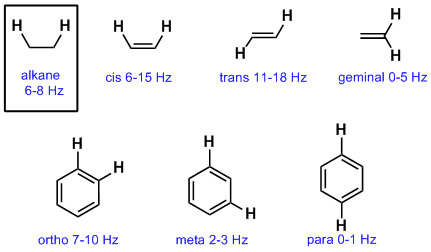
Vinylic c h bond is lower.
Vinylic carbon can have either two double bonds in its sides or one double bond.
It is the ethylene molecule with one fewer hydrogen atom.
The vinyl compounds are every reactive and they polymerize to form the vinyl polymers as in the case of polyvinyl acetate polyvinyl chloride and polyvinyl fluoride.
Vinylic carbon can have only two carbon as the maximum number.
In chemistry vinyl or ethenyl is the functional group with the formula ch ch2.
The general formula for vinyl group is r ch ch 2 in which both carbon atoms are bonded with double bond and r is attached at vinylic position.