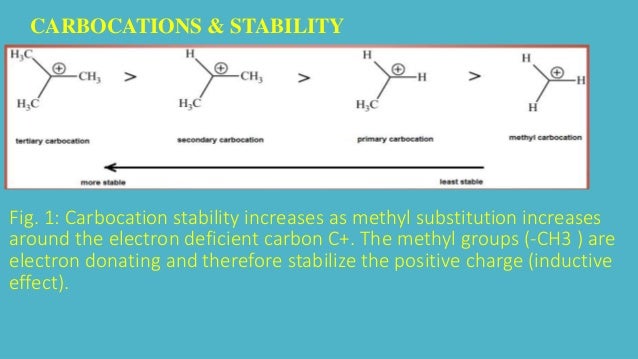
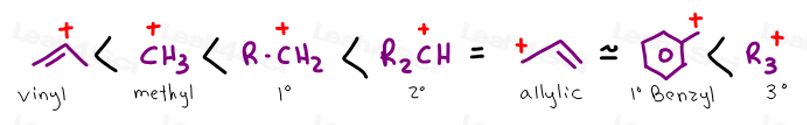
Any trivalent disubstituted carbon is generally a vinylic carbocation in which the carbon atom which is bearing the positive charge is found to be double bonded and will always exist as sp hybridized.
Vinylic carbocation definition.
We can basically say that they are carbon cations.
The vinyl cation is a carbocation with the positive charge on an alkene carbon.
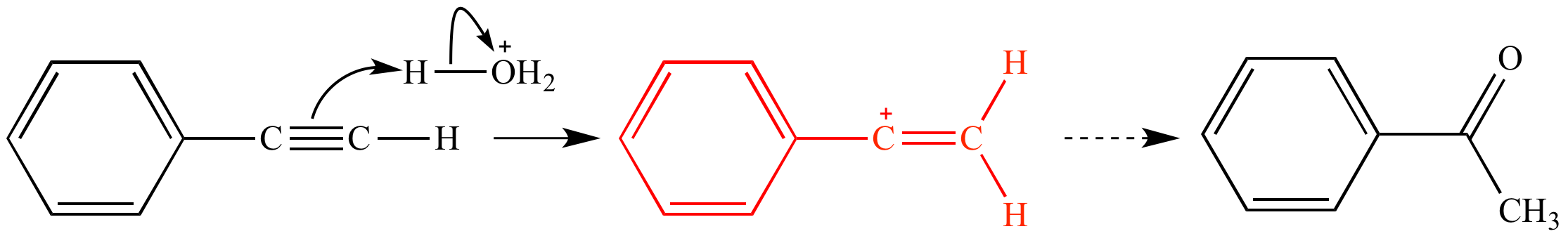
Acid catalyzed hydration of phenyl acetylene a terminal alkyne involves a vinylic carbocation intermediate.
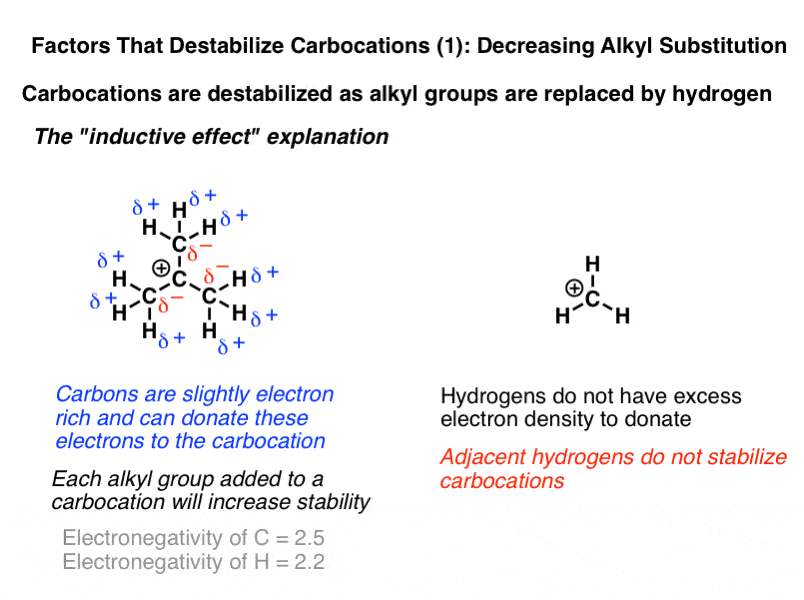
For a positive center to be attached to a more electronegative group is destabilizing.
Formerly it was known as carbonium ion.
In the first mechanism step the alkyne is protonated by hydronium ion a strong acid to produce a resonance stabilized secondary vinylic carbocation shown in red.
The univalent hydrocarbon group ch 2 ch derived from ethylene.
Look at the figure below notice that in the alkyl carbocation on the left the cationic center is attached to an s p x 3 carbon whereas in the vinylic cation in the middle the cationic center is attached to a more electronegative s p x 2 carbon.
In the absence of geometric constraints most substituted vinyl cations carry the formal positive charge on an sp hydridized carbon atom of linear geometry.
Atoms or groups attached to an allylic carbon are termed allylic substituents.
This carbocation is also a benzylic carbocation.
Its empirical formula is c 2h 3.
A carbocation is a molecule in which a carbon atom has a positive charge and three bonds.
An allylic carbon is an sp3 carbon that is adjacent to a vinylic carbon.
A carbocation with a two coordinate positive carbon derived from formal removal of a hydride ion h from an alkene is known as a vinyl cation.
Carbocation today is defined as any even electron cation that possesses a significant positive charge on the carbon atom.